Agricultural Literacy Curriculum Matrix
Lesson Plan
FoodMASTER Middle: Energy Balance
Grade Level
Purpose
Students will identify the importance of a healthy diet, examine how to meet current Dietary Guidelines, and determine the potential energy (kilocalories) of a peanut through measurements obtained during teacher use of a bomb calorimeter. Students will make comparisons to the actual Nutrition Fact Label and identify possible sources of error. Grades 6-8
Estimated Time
Materials Needed
Engage:
- Magnificent Menu student handout, 1 per student
Lab:
Teacher Materials, demonstration (see step 6 in lab procedures)
- Safety goggles
- Apron
- 1 empty soda can
- 1 empty metal coffee can (large enough to fit a soda can inside)
- 1 cork
- 1 uncoated paper clip (any size)
- 1 graduated cylinder
- 100mL distilled water (room temperature)
- 1 thermometer (Celsius)
- 1 lighter
- 1 forceps
- 1 peanut for calorimeter
- 1 glass or metal rod (16cm or longer)
- 1 can opener with a triangular end (church key)
Student Materials, per group of 4-5 students
- Energy Equilibrium student handout, 1 per student (Key)
- Energy Balance lab sheet, 1 per student (Key)
- 1 small bowl 1 graduated cylinder or liquid measuring cup
- 1 triple beam balance
- 100mL distilled water (room temperature)
- 1 cup of peanuts
Vocabulary
bomb calorimeter: a vessel for measuring heat of combustion by igniting a sample
calorie: a unit of heat energy; the amount of heat required to raise the temperature of one gram of water one degree Celsius; used to indicate the amount of energy that foods will produce in the human body
energy balance: the biological homeostasis of energy in a living system; relation between intake of food and output of work; when balance is positive, the body stores extra energy as fat; when balance is negative, the body uses stored energy (fat), resulting in weight loss
joule: a unit of work or energy equal to the work done by a force of one newton
kilocalorie: the standard unit used to describe the amount of energy that foods will produce in the human body and is reported on Nutrition Facts Labels
Did You Know?
- A calorie is a unit of measurement — but it doesn't measure weight or length. A calorie is a unit of energy.1
- Calories first began being used as a unit of measurement in food in the 1800s.2
- A gram of fat usually contains about 9 calories. A gram of carbohydrate or protein contains 4 calories.3
Background Agricultural Connections
The Importance of Meal Planning for a Healthy Diet
Meal planning is an important part of creating a healthy diet. For good health, people should plan weekly meals to help them make good choices. Each day adolescents should eat 1 1/2 cups of fruit, 2 1/2 cups of vegetables, 6 ounces of grains (1/2 of them being whole grains), 5 ounces of protein, and 3 cups of dairy. Eating the recommended amounts of each food group may help to prevent disease and support the body with nutrients. Eating too many calories from unhealthy foods can put individuals at risk for becoming overweight, developing dental cavities, and experiencing heart problems, diabetes, high blood pressure, and other diseases. In this lesson, students will learn about the calorie as the unit of energy we obtain from food, and how to interpret health information from a variety of sources. These topics are important to help students understand nutritional concepts.
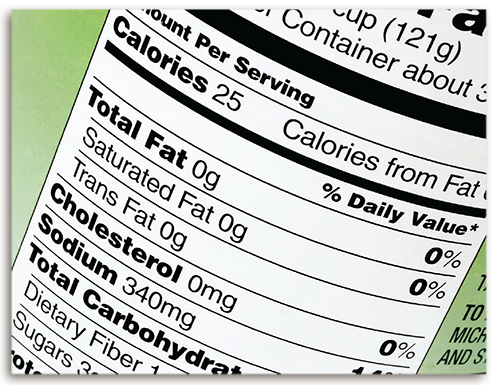
Understanding Energy from Food
Our bodies need food for energy. Some foods provide more energy than others. More specifically, some foods are better sources of energy because they allow our bodies to store more potential energy. Potential energy is stored energy that has not been used. The amount of potential energy varies among foods. Our bodies convert the potential energy, measured in calories, to chemical energy. Chemical energy is the energy stored in chemical bonds that is released during a chemical reaction. A calorie is the amount of energy needed to raise one milliliter of water one degree Celsius. A kilocalorie (kcal) is the amount of energy required to raise the temperature of one liter of water one degree Celsius. Kilocalories are also known as the calories seen on food labels. They are a good method to use when measuring our energy. Joules is another unit used to measure energy intake. A joule (J) is equal to the amount of energy expended to force one newton through one meter. Just like ounces and grams are units used to measure weight, calories and joules are units used to measure energy.
It is important to consume the right amount of energy. Everyone burns energy at their own rate; however, we can calculate a range of calories we need for daily functions. We can keep track of our energy consumption using the energy balance equation. This equation subtracts energy burned from energy consumed to determine if we have consumed too little or too much energy. Consistently consuming too much or too little energy will have lasting effects on our bodies. Consuming too little energy will cause our bodies to breakdown too much stored energy, leading to malnutrition. Consuming too much energy will cause our bodies to store too much energy, leading to obesity. We need to consume a balanced diet to ensure the best health outcomes. Selecting foods from all five food groups, according to the daily recommendations for our age and gender, is essential to our health. The five food groups include Dairy, Grains, Protein, Vegetables, and Fruit. They provide us with a well-rounded diet based on our macronutrient recommendations. Macronutrients are carbohydrates, protein, and fat. We need all three to keep our bodies functioning properly. Selecting too many energy-dense foods and too little nutrient-dense foods will lead to health problems.
Energy dense foods, like doughnuts and French fries, are foods high in calories and low in nutrients. Nutrient dense foods, like broccoli and strawberries, can be high or low in calories, but are always high in nutrients. A bomb calorimeter can be used to measure the energy in food. This tool measures the amount of heat generated by a chemical reaction by releasing the energy from food in the form of heat. For example, when determining the amount of energy in a peanut, the peanut is placed in the calorimeter and burned. As the peanut is burning, its energy is being transferred to the water sitting above in the form of heat; therefore the increase in water temperature can be used to determine the peanut’s energy.
FoodMASTER Middle Lessons
FoodMASTER (Food, Math and Science Teaching Enhancement Resource) is a compilation of programs aimed at using food as a tool to teach mathematics and science. For more information see the Background & Introduction to FoodMASTER for Middle School. This lesson is one in a series of lessons designed for middle school:
Engage
- Conduct a short class discussion by asking students what health benefits come from consuming a balanced diet. Students should seek to identify specific food groups, their health benefits, and common food sources of each.
- Give each student one copy of the Magnificent Menu student handout. If completed in-class, allow students to work in small groups on Investigation worksheet to further explore the topic and respond to questions.
- Follow-up with a class discussion about student findings related to the health benefits of consuming a balanced diet and student generated ideas for making their own diet “balanced.”
Explore and Explain
Lab: Energy Balance
Teacher Preparation:
- Review information found in the Background Agricultural Connections section of the lesson, lesson Procedures, and the attached Essential Files.
- Prepare student materials for each group. If time is a concern, construct the bomb calorimeter before hand and pre-weigh the single peanut.
- Note the following:
- The mathematics within this lesson may be challenging to some students. Consider guiding students through the mathematics portion of the lab, or assigning one student within each group to lead others through the work.
- A portion of this lab investigation (burning of food) should occur in an open area outside unless you have access to a fume hood in your classroom.
- WARNING: Students with peanut allergies may not be able to participate in this investigation.
- Construction of the of the Bomb Calorimeter:
- Choose one of the following methods:
- Option 1: Use the triangular end of a can opener to put four holes evenly spaced around the bottom edge of the coffee can. One hole should be large enough to fit a peanut through.
- Option 2: Remove the bottom of an open coffee can.
- Drill or poke holes (the size of a pencil eraser or smaller) through a small aluminum can (soda) opposite each other about 1 cm below the top. Do not remove the top. The water and thermometer can be placed into the can through the drinking opening.
- Insert a glass or metal rod, 16cm or longer through the holes you made at the top of the soda can. This rod will support the can when it is placed across the top of the coffee can.
- Note: If using Option 1 construction, placing the soda can on the base of the coffee can instead of hanging it should provide a sufficient temperature increase.
- The bomb calorimeter is now ready for the lab. See step 6 of the lab procedures below for further steps.
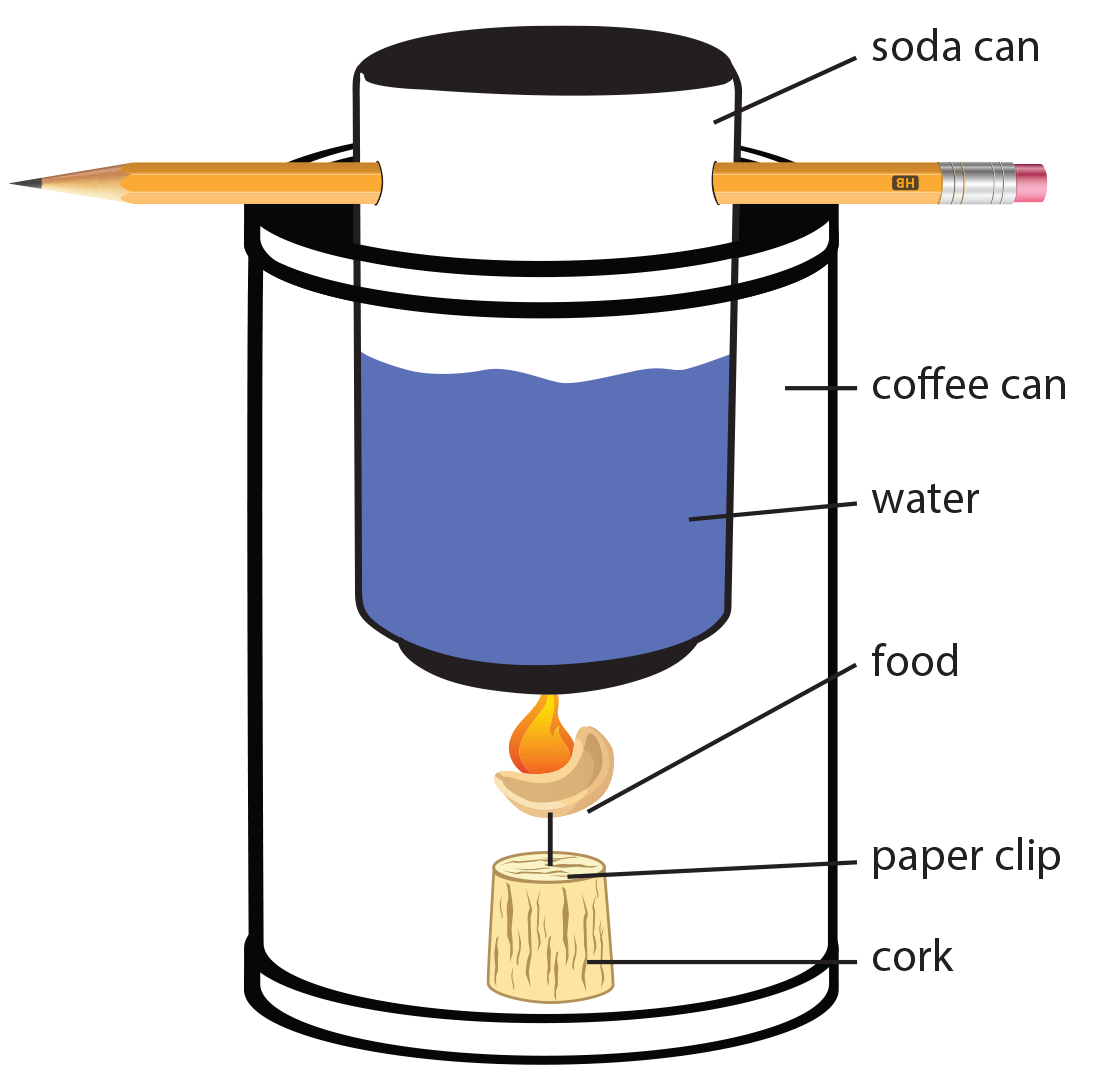
- Choose one of the following methods:
Procedures:
- Consider having your students research kilocalories as a unit of measuring energy in food prior to beginning the lab investigation.
- Distribute lab materials. It is recommended that materials are organized into stations for easier distribution. Students should be arranged in small groups of 4-5. Each group should receive the lab supplies outlined in the Materials section as well as 1 copy of the Energy Balance lab sheet and one copy of the Energy Equilibrium student handout.
- Ask students to read Energy Equilibrium and complete the "Think About It" questions for this lab investigation.
- Before beginning the lab investigation:
- Require students to wash their hands.
- Emphasize the importance of practicing good food safety behaviors by not consuming substances used as part of the lab investigation
- Launch the lab by asking students to observe and make a prediction about the kilocalorie content of peanuts.
- Next, complete the teacher demonstration or show the provided video lab demonstration, Food Explorations Lab: Energy Balance. If you use the video, make sure students record the measurement values provided in the appropriate tables. If performing the demonstration in class, follow these steps:
- Begin by weighing and recording the mass of a single peanut. This same peanut will later be placed into the bomb calorimeter. Students should record the weight in Table C. If you completed this step ahead of time, simply provide students with the weight of the single peanut.
- Next, place 100mL of room temperature distilled water in the soda can.
- Place the soda can inside of the coffee can.
- Unwrap the paper clip and insert it in the cork.
- Wrap the paper clip attached to the cork around the peanut. If the peanut breaks off while it is burning, you will have to burn a new peanut. Be sure to re-weigh the peanut each time.
- Light the peanut with a wooden match or lighter. Once it ignites, immediately place the coffee can around the burning peanut or insert it through a hole in the bottom of the calorimeter.
- Make sure the peanut burns completely. If the fire goes out, re-light the peanut.
- WARNING: If you have to relight the peanut, be careful to use caution when touching the heated coffee can.
- Once the peanut has completely burned, stir the water with the thermometer and measure the temperature. Both cans may be warm. Be careful not to burn yourself.
- After the burned peanut has cooled, measure and record its weight. Students should record the weight in Table C.
-
Allow students to work in small groups to complete the Energy Balance lab sheet and respond to lab questions. Note that if you used a thermometer that measured in Fahrenheit instead of Celsius, before proceeding with the conclusion questions you will need to help students convert degrees Fahrenheit (°F) to degrees Celsius (°C). You may consider completing this math for students or guiding them through the conversion. An example of the °F to °C conversion, as it relates to conclusion question #1, is provided for you below. The water temperatures provided in the equation below are for example purposes only.
(5/9) (70°F (ΔT) − 32) = 21.11°C
(5/9) (71°F (ΔT) − 32) = 21.67°C
(21.67°C after burning peanut − 21.11°C before burning peanut) = 0.56°C water (ΔT)
100 g water × 1 cal/g/°C × 0.56°C = 56 cal (Q) or 0.056 kcal
- Follow-up with a class discussion about the relationship between human health and consuming nutrient dense (foods high in nutrients, low in calories - e.g. vegetables/ fruits) versus calorie dense foods (foods high in calories, low in nutrients - e.g. French fries, soda). See Elaboration Activities for ideas on how to further extend this lesson.
Elaborate
-
Explore the energy density of other foods by using the bomb calorimeter or reviewing Nutrition Facts labels.
-
Use the Managing your Meals student handout (Key) fo review meal management and for a student recall of the foods they eat.
Evaluate
After conducting these activities, review and summarize the following key concepts:
- Potential energy is stored energy that has not been used. The amount of potential energy varies among foods.
- A calorie is the amount of energy needed to raise one milliliter of water one degree Celsius. The energy contained in the food we eat is measured in calories.
- Consuming too little energy will cause our bodies to breakdown too much stored energy, leading to malnutrition. Consuming too much energy will cause our bodies to store too much energy, leading to obesity. We need to consume a balanced diet to ensure the best health outcomes.
Sources
- http://kidshealth.org/en/kids/calorie.html
- http://articles.latimes.com/2010/feb/15/health/la-he-calories-about15-2010feb15
- https://www.nal.usda.gov/fnic/how-many-calories-are-one-gram-fat-carbohydrate-or-protein
Acknowledgements
This lesson was partnered with East Carolina University. The FoodMASTER program was supported by the Science Education Partnership Award (SEPA) which is funded from the National Center for Research Resources, a component of the National Institutes of Health.
- Primary Authors:
- Virginia Stage, PhD, RDN, LDN
- Mary White
- Ashley Roseno, MAEd, MS, RDN, LDN
- Melani W. Duffrin, PhD, RDN, LDN
- Graphic Design: Cara Cairns Design, LLC
Recommended Companion Resources
Author
Organization
| We welcome your feedback! If you have a question about this lesson or would like to report a broken link, please send us an email at matrixelearning@gmail.com. If you have used this lesson and are willing to share your experience, we will provide you with a coupon code for 10% off your next purchase at AgClassroomStore. |
